Applying Conversion Factors to Stoichiometry
Now you're ready to use what you know about conversion factors to solve some stoichiometric problems in chemistry. Almost all stoichiometric problems can be solved in just four simple steps:
- Balance the equation.
- Convert units of a given substance to moles.
- Using the mole ratio, calculate the moles of substance yielded by the reaction.
- Convert moles of wanted substance to desired units.
These "simple" steps probably look complicated at first glance, but relax, they will all become clear.
Let's begin our tour of stoichiometry by looking at the equation for how iron rusts:
Step 1. Balancing the Equation
The constituent parts of a chemical equation are never destroyed or lost: the yield of a reaction must exactly correspond to the original reagents. This fact holds not just for the type of elements in the yield, but also the number. Given our unbalanced equation:
This equation states that 1 iron (Fe) atom will react with two oxygen (O) atoms to yield 2 iron atoms and 3 oxygen atoms. (The subscript number, such as the two in
O2 describe how many atoms of an element are in a molecule.) This unbalanced reaction can't possibly represent a real reaction because it describes a reaction in which one Fe atom magically becomes two Fe atoms.
Therefore, we must balance the equation by placing coefficients before the various molecules and atoms to ensure that the number of atoms on the left side of the arrow corresponds exactly to the number of elements on the right.
Let's count up the atoms in this new, balanced version of the reaction. On the left of the arrow we have 4 atoms of iron and 6 atoms of oxygen (since
3×2 = 6 ). On the right we also have 4 iron (since
2×2 = 4 ) and 6 oxygen (
2×3 = 6 ). The atoms on both sides of the equation match.
The process of balancing an equation is basically trial and error. It gets easier and easier with practice. You will likely start to balance equations almost automatically in your mind.
Step 2. Converting Given Units of a Substance to Moles
The process of converting given units into moles involves conversion factors. Below we will provide the most common and important conversion factors to convert between moles and grams, moles and volumes of gases, moles and molecules, and moles and solutions. These conversion factors function in the same way as those discussed in the previous section Note also that though these conversion factors focus on converting from some other unit to moles, they can also be turned around, allowing you to convert from moles to some other unit.
Converting from Grams to Moles
The gram formula mass of a compound (or element) can be defined as the mass of one mole of the compound. As the definition suggests, it is measured in grams/mole and is found by summing the atomic weights of every atom in the compound. Atomic weights on the periodic table are given in terms of amu (atomic mass units), but, by design, amu correspond to the gram formula mass. In other words, a mole of a 12 amu carbon atom will weigh 12 grams.
The gram formula mass can be used as a conversion factor in stoichiometric calculations through the following equation:
Moles = 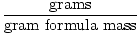 | |
Gram formula mass is also known as GFM. You may also see the term gram molecular mass, abbreviated GMM. This term is often used instead of GFM when the substance is molecular and not ionic. However, only the terminology is different, GMM is used in the same way as GFM. Therefore, I will use the catch- all term GFM in this study guide.
Converting between Volume of a Gas and Moles
The Ideal Gas law, discussed at length in the Sparknote on Gases, provides a handy means of converting between moles and a gas, provided you know certain qualities of that gas. The Ideal Gas Law is PV = nRT , withn representing the number of moles. If we rearrange the equation to solve for n , we get:
n = 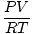 | |
with
P representing pressure in atm,
V representing volume in liters,
T representing temperature in Kelvins, and
R the gas constant, which equals .0821 L-atm/mol-K. Given
P ,
V , and
T , you can calculate the number of moles of substance in a gas.
In those instances when a problem specifies that the calculations are to be made at STP (Standard Temperature and Pressure; P = 1 atm, T = 273 K)), the problem becomes even simpler. At STP, a mole of gas will always occupy 22.4 L of volume. If you are given a volume of a gas at STP, you can calculate the moles in that gas by calculating the volume you are given as a fraction of 22.4 L. At STP, 11.2 L of a gas will be .5 moles; 89.6 L of gas will be 4 moles.
Converting between Individual Particles and Moles
Avogadro's Number provides the conversion factor for moving from number of particles to moles. There are 6.02×1023 formula units of particles in every mole of substance, with formula unit describing the substance we are looking at, whether it is a compound, molecule, atom, or ion. A formula unit is the smallest unit of a substance that still retains that substance's properties and is the simplest way to write the formula of the substance without coefficients. Some representative formula units are listed below.
- Compounds: Cu2S , NaCl
- Molecules: N2 , H2
- Atoms: Fe, Na
- Ions: Na+(aq) , Cl-(aq)
Since
1 mole = 6.02×1023 formula units, the conversion from formula units to moles is simple:
Moles = 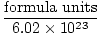 | |
Converting between Solutions and Moles
Solutions are discussed in much greater detail in the series of Solutions SparkNotes. But it is possible, and fairly easy to convert between the measures of solution (molarity and molality) and moles.
Molarity is defined as the number of moles of solute divided by the number of liters of solvent. Rearranging the equation to solve for moles yields:
Moles = molarity × liters of solution
MolaLity is defined as the number of moles of solute divided by the number of kilograms of solvent. Rearranging the equation to solve for moles yields:
Moles = molality × kilograms of solution
Using the Mole Ratio to Calulate Yield
Before demonstrating how to calculate how much yield a reaction will produce, we must first explain what the mole ratio is.
The Mole Ratio
Let's look once again at our balanced demonstration reaction:
The coefficients in front of iron, oxygen, and iron (III) oxide are ratios that govern the reaction; in other words, these numbers do not demand that the reaction can only take place with the presence of exactly 4 moles of iron and 3 moles of oxygen, producing 2 moles of iron (III) oxide. Instead, these numbers state the ratio of the reaction: the amount of iron and oxygen reaction together will follow a ratio of 4 to 3. The mole ratio describes exactly what its name suggests, the molar ratio at which a reaction will proceed. For example, 2 moles of Fe will react with 1.5 moles of O2 to yield 1 mole of Fe2O3 . Alternatively, 20 moles of Fe will react with 15 moles ofO2 to yield 10 moles of Fe2O3 . Each of these examples of the reaction follow the 4:3:2 ratio described by the coefficients.
Now, with a balanced equation, the given units converted to moles, and our understanding of the mole ration, which will allow us to see the ratio of reactants to each other and to their product, we can calculate the yield of a reaction in moles. Step 4 demands that we be able to convert from moles to back to the units requested in a specific problem, but that only involves turning backwards the specific converstion factors described above.
Sample Problems
Problem: Given the following equation at STP:
Determine what volume of H
2(g) is needed to produce 224 L of NH
3(g).
Solution:
br> Step 1: Balance the equation.
Step 2: Convert the given quantity to moles. Note in this step, 22.4 L is on the denominator of the conversion factors since we want to convert from liters to moles. Remember your conversion factors must always be arranged so that the units cancel.
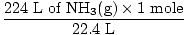 = 10 moles of NH3(g) = 10 moles of NH3(g) | |
Step 3: mole ratio.
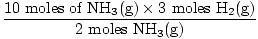 = 15 moles H2(g) = 15 moles H2(g) | |
Step 4, convert to desired units:
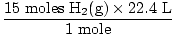 = 336 L H2(g) = 336 L H2(g) | |
Now for a more challenging problem:
Given the following reaction:
| 2H2S(g) + O2(g)→SO2(g) + 2H2O(s) | |
How many atoms of oxygen do I need in order to get 18 g of ice?
Solution
Step 1. The equation is partially balanced already, but let's finish the job.
| 2H2S(g) +3O2(g)→2SO2(g) + 2H2O(s) | |
Step 2, convert to moles:
1 formula unit of H2O has 2 atoms of H and 1 atom of O
The atomic mass of H is 1 gram/mole
Atomic mass of O = 16 grams/mole
GFM of H2O(s) = 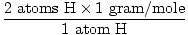 + + 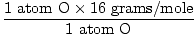 = 18 grams / mole = 18 grams / mole | |
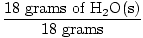 ×1 mole = 1 mole of H2O(s) ×1 mole = 1 mole of H2O(s) | |
Step 3, mole ratio:
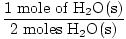 ×3 moles O2(g) = 1.5 moles O2(g) ×3 moles O2(g) = 1.5 moles O2(g) | |
Step 4, convert to desired units:
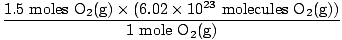 = 9.03×1023 molecules O2(g) = 9.03×1023 molecules O2(g) | |
Is this the answer? No. The question asks for ATOMS of oxygen. There are two atoms of oxygen in each molecule of O2(g).
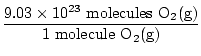 ×2 atoms O = 1.806×1024 atoms O ×2 atoms O = 1.806×1024 atoms O | |
Now we're done. Note how important it was to write out not only your units, but what substance you're currently working with throughout the problem. Only a brief check was needed to ascertain if we were really answering the given question. Always check to make sure you have answered the correct question.
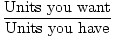
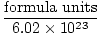
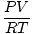
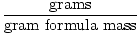
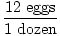
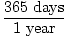
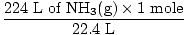 = 10 moles of NH3(g)
= 10 moles of NH3(g)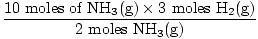 = 15 moles H2(g)
= 15 moles H2(g)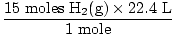 = 336 L H2(g)
= 336 L H2(g)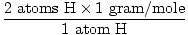 +
+ 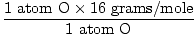 = 18 grams / mole
= 18 grams / mole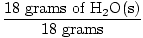 ×1 mole = 1 mole of H2O(s)
×1 mole = 1 mole of H2O(s)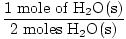 ×3 moles O2(g) = 1.5 moles O2(g)
×3 moles O2(g) = 1.5 moles O2(g)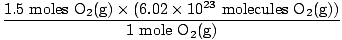 = 9.03×1023 molecules O2(g)
= 9.03×1023 molecules O2(g)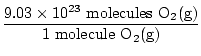 ×2 atoms O = 1.806×1024 atoms O
×2 atoms O = 1.806×1024 atoms O