Introduction
Acids and bases play a central role in chemistry because, with the exception of redox reactions, every chemical reaction can be classified as an acid-base reaction. Our understanding of chemical reactions as acid-base interactions comes from the wide acceptance of the Lewis definition of acids and bases, which supplanted both the earlier Bronsted-Lowry concept and the first definition--the Arrhenius model. Arrhenius first defined acids as proton (H+) producers in aqueous solution and bases as hydroxide (OH-) producers. Although this model is intuitively correct, it is limited to substances that include proton and hydroxide groups. Bronsted and Lowry proposed the more general definitions of acids and bases as proton donors and acceptors, respectively. Unlike the Arrhenius conception, the Bronsted-Lowry model accounts for acids in solvents other than water, where the proton transfers do not necessarily involve hydroxide ions. But the Bronsted-Lowry model fails to explain the observation that metal ions make water more acidic (discussed in Calculating pH's). Finally, Lewis gave us the more general definition of acids and bases that we use today. According to Lewis, acids are electron pair acceptors and bases are electron pair donors. Any chemical reaction that can be represented as a simple exchange of valence electron pairs to break and form bonds is therefore an acid-base reaction.
Acid-base chemistry is important to us on a practical level as well, outside of laboratory chemical reactions. Our bodily functions, ranging from the microscopic transport of ions across nerve cell membranes to the macroscopic acidic digestion of food in the stomach, are all ruled by the principles of acid-base chemistry. Homeostasis, the temperature and chemical balances in our bodies, is maintained by acid-base reactions. For example, fluctuations in the pH, or concentration of hydrogen ions, of our blood is moderated at a comfortable level through use of buffers. Learning how buffers work and what their limitations are can help us to better understand our physiology. We will start by introducing fundamentals of acid-base chemistry and the calculation of pH, and then we will cover techniques for measuring pH. We learn about buffers and see how they are applied to measure the acidic content of solutions through titration.
Terms
Fundamentals of Acids and Bases
To achieve our goal of understanding how acids and bases work, we must first define what acids and bases are. There are three distinct conceptions of acids and bases that will be considered in this SparkNote--the Arrhenius model, the Bronsted-Lowry model, and the Lewis model. Each of these models describe an acid-base reaction as a process by which a transfer occurs between two partner reagents, the acid and the base. For our purposes, the most useful model is the Bronsted-Lowry model, because we will be considering reactions involving proton transfers. Bronsted and Lowry described acids as proton donors and bases as proton acceptors. Calculations and measurements of pH are relevant to the Bronsted-Lowry conception of acid-base reactions. We will discuss each of the three models of acid-base reactions using representative equations.
To speak of acid or base strength, we need scales for acidity and basicity. pH and pOH scales are quantitative representations of these values for acidic and basic solutions. Next, we will define the acid dissociation constant, K a, and the base dissociation constant, K b, to quantify the strengths of particular acids and bases. These terms allow us to process acid and base strength mathematically and package them into values that we can gage conceptually. Using reference values, we can then see that a solution with pH 6 is weakly acidic, since it is slightly lower in pH than water at pH 7.
Terms
Fundamentals of Acid-Base Chemistry
Definitions of Acidity and Basicity
For more than two-hundred years, chemists have struggled to come up with a way to describe acid-base reactions that is at the same time physically relevant, specific enough to be accurate, and general enough to include everything that should be considered an acid-base relationship.
Svante Arrhenius first defined acids to be proton (H+) donors and bases to be hydroxide ion (OH-) donors in aqueous solution. The Arrhenius model of acids and bases is summarized by the following two reactions:
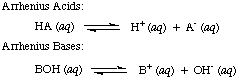
At the time that Arrhenius proposed these definitions, water was virtually the only solvent used in chemistry, and nearly all known acids and bases contained protons (H+) and hydroxyl groups (OH), respectively. His definition was sufficient for the chemistry that was understood then. But progress in chemistry necessitated new definitions: it was discovered that ammonia behaves like a base, and HCl donates protons in non-aqueous solvents. The Bronsted-Lowry model of acids and bases serves that need by describing acids as proton donors and bases as proton acceptors. These definitions remove the role of solvent and allow bases like ammonia and fluoride ion to be classified as bases, so long as they bond to protons. The Bronsted-Lowry model implies that there is a relationship between acids and bases (acids transfer protons to bases) and allows us to define conjugate acids and conjugate bases, as seen in .
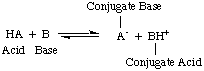
You should note in the figure above that the conjugate acid of the base, BH+, acts as an acid in the reverse reaction by donating a proton to the conjugate base, A-, of the acid HA.
Despite the usefulness of the Bronsted-Lowry definition, there is an even more general definition of acids and bases provided by G. N. Lewis. The Lewis model of acids and bases proposes that an acid is an electron pair acceptor while a base is an electron pair donor. This model of acidity and basicity broadens the characterization of acid-base reactions to include reactions like the following which do not involve any hydrogen transfers. The nitrogen atom in ammonia donates an electron pair to complete the valence octet of boron.
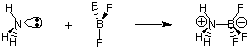
Because we are more interested now in describing terms and processes that involve proton transfers (pH, titration), we will focus on the Bronsted-Lowry definitions of acids and bases. We will leave consideration of the Lewis model of acids and bases for studying reactions in organic chemistry.
Reactions of Acids and Bases with Water
As you may have noted already in the acid-base reactions above, we use arrows in both reaction directions to indicate that these are equilibrium processes. Proportions of reagents and products at equilibrium can be described by an equilibrium constant. The equilibrium constant given in is for the reaction of an acid, HA, with water as shown.
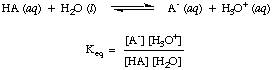
Although water is a reactant in the above reaction and belongs in the equilibrium constant, its value of 55.6 M in aqueous solution is so large in comparison with the change in water concentration at equilibrium that we will assume that the value of [H2O] is constant. Using that assumption, we will define the acid dissociation constant, K a, in to be the following:
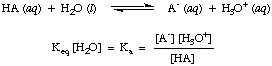
From the form of the above equation we can see that stronger acids, those that dissociate to a greater extent, will have larger values of K a whereas weaker acids will have smaller values of K a. A practical range for K avalues runs from 10-12 for very weak acids to 1013 for the strongest acids. Knowing this practical range of acidity constants will aid in judging how reasonable your answers are when you calculate values for K a in problems.
In an analogous way, we define K b, the base constant in to be the following:
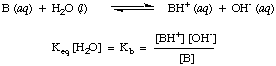
Stronger bases have larger values of K b while weaker bases have smaller values of K b. K b's of typical bases in inorganic chemistry tend to have a range of values between 10-11 and 103.
As you may have discovered in our above discussion, water can act as both an acid and as a base. For this reason water is said to be amphiprotic. Water is often incorrectly termed amphoteric. An amphiprotic species like water can either donate or accept a proton. Amphoteric species can both donate and accept hydroxide ions, as water cannot. The following reaction in , called the autoionization of water, has the equilibrium constant K wdefined in the manner of K a and K b. The dissociation constant K w for water is 1 x 10-14 at room temperature (298 K), and tends to rise with higher temperatures.
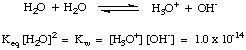
Knowing that K w = 1 x 10-14 is useful because the relationship between Ka and K b for a conjugate acid-base pair is K a * K b = K w. Therefore, you can calculate the K a of the conjugate acid of a base when given its K b. This point is proved in below.
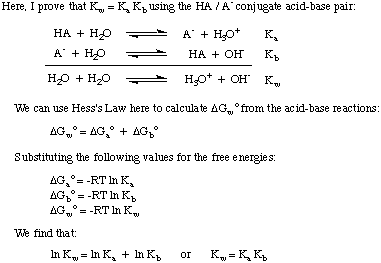
From the proven expression, we see that strong acids (large K a's) form weak conjugate bases and weak acids form strong conjugate bases. In the following section, we will discuss acid and base strength in more depth.
Strengths of Acids and Bases
The pH and pOH Scales of Acidity
Due to the large range of proton concentration values ([H+]) in aqueous solution (typically from 10-15 to 10 M), a logarithmic scale of acidity makes the most sense to put the values into manageable numbers. The pH scale of acidity defines pH as the negative common logarithm of the concentration of H+: pH = - log [H+].
Before proceeding with our discussion on pH, let's review some properties of Logarithms (affectionately called "logs"). A common logarithm is a function that computes what exponent would be on 10 to obtain the input number. For example, the logarithm of 100 (written "log 100") is 2 because 102 = 100. Likewise, log 1,000,000 = 6. To add logs, multiply their arguments: log 100 + log 1000 = log (100 x 1000) = log (100,000) = 5. To subtract logs, divide their arguments: log 100 - log 1000 = log (100/1000) = log (0.1) = - 1. Given those simple rules, you can now manipulate logs well enough to do any problem involving logs in acid-base chemistry. For this section of chemistry, you will get to know and love the log key on your calculator almost as much as you did in kinetics.
The pH scale in water is centered around 7, which is called neutral pH. This value is not randomly selected, but rather it comes from the fact that the [H+] in pure water is 10-7 (recall that K w = 10-14). An acidic solution has a pH value less than 7 because [H+] is greater than that of pure water. A basic solution has a pH greater than 7 because there is a lower [H+] than that of pure water (and consequently a larger [OH-]).
Chemists use a pOH scale analogous to the pH acidity scale to gage hydroxide ion concentrations of aqueous solutions. pOH is defined as the negative common logarithm of the concentration of OH-: pOH = - log [OH- ]. In the pOH scale, 7 is neutral, less than 7 is basic, and greater than 7 is acidic. A useful relationship (which you should be able to derive using the definition of K w) is that pH + pOH = 14. This formula will allow you to readily convert between values of pH and pOH. A comparison of the pH and pOH scales is provided in . Note that because K w is constant, the product of [H+] and [OH-] is always equal to 10-14.

As you have probably guessed by now, the prefix "p" in front of a symbol means "take the negative log". We have defined pH and pOH as values to describe the acidic or basic strength of solutions. Now we can talk about pK a (- log K a) and pK b (- log K b) as measures of acidity and basicity, respectively, for standard acids and bases. An acid with a pK a less than zero is called a strong acid because it almost completely dissociates in water, giving an aqueous solution a relatively low pH. Acids with pK a's greater than zero are called weak acids because they only partially dissociate in water, to make solutions with larger pH's than those strong acids produce at the same concentration. Similarly, bases with pK b's less than zero are strong bases and bases with pK b's greater than zero are called weak bases.
Factors Affecting Acidity
Now that we have the tools to be able to describe the acidity and basicity of compounds with known pK a's, let's analyze trends in acidity and basicity to reach an intuitive understanding of those trends.
First, let's consider the acidity of the halogen acids--HF, HCl, HBr, and HI-- collectively abbreviated HX as shown in , where X represents the halogen. From the data in the figure below, you can see that the major factor affecting the acidity of halogen acids is the strength of the H-X bond. Intuitively, a larger electronegativity difference should lead to a stronger acid due to the polarization of electrons away from hydrogen. However, the trend in bond strength is enough to overrule that competing trend in electronegativity. The smaller the halogen, the closer in size it is to the proton and the greater the orbital overlap; so HF is most the strongly bonded and weakly acidic of the halogen acids.
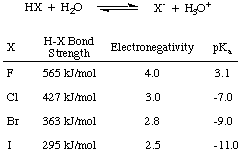
Generalizing that result, we can say that when the an H-A bond is strong, the acid is weak. Experimental confirmation of this postulate comes from the oxyacid series of compounds. An oxyacid is a molecule of the form AOn(OH)m, where A is a non-metal. Pauling and Ricci derived the following approximate equation for oxyacid acidity from experimental observations:
pK a = 8 - 9f + 4n
The variable f is the formal charge on A when all oxygens are singly bound to A. The variable n represents the number of O atoms bound to A that are not bound to an H. A general trend, summarized in the Pauling-Ricci rule above, is that the more electron withdrawing (more electropositive) the non- metal center, the stronger the acid due to a weakening of the O-H bond.
In summary, we note the following trend regarding acidity: hydrogens are more weakly bound to more electronegative groups, and this produces stronger acids. By using the relationship K w = K a * K b, you should be able to figure out that we need only to discuss the acidity of compounds to describe basicity. From the above discussion, we can deduce that bases with weaker conjugate acids are more basic than those with stronger conjugate acids. Therefore, bases that form stronger bonds to H will have larger K b's and are stronger bases.




As a global Contract Research Organization (CRO), headquartered in New York, USA, Alfa Chemistry has served the pharmaceutical and biotechnology industries for eight years. ACID YELLOW 99
ReplyDelete