Covalent Bonding
A covalent bond represents the sharing of an electron pair between atoms. By sharing electrons, as we first described it in Properties of Bonds, each atom in a bond can achieve stability by having an octet of valence electrons. To keep track of the electrons surrounding each atom in a covalent bond, we will introduce Lewis structures. When drawing Lewis structures we will learn how to calculate the charges on individual atoms in molecules by using the concept of formal charge.
Often, when drawing Lewis structures we forget that molecules are real things with three dimensional shapes. To describe the shapes of molecules we will use Valence Shell Electron Pair Repulsion theory (VSEPR). As we know from Coulomb's law (in the SparkNote on ionic bonding), electrons repel each other. VSEPR simply states that the most stable shape for a molecule will be the one that places the electrons farthest apart. In this section, we will fully explore the consequences of VSEPR for the shapes of the common types of molecules you will encounter.
Terms
The Covalent Bond
Lewis Structures
A covalent bond represents a shared electron pair between nuclei. The Stability of covalent bonds is due to the build-up of electron density between the nuclei. Using Coulomb's law (discussed in Ionic Bonding), you should note that it is more stable for electrons to be shared between nuclei than to be near only one nucleus. Also, by sharing electron pairs nuclei can achieve octets of electrons in their valence shells, which leads to greater stability.
To keep track of the number and location of valence electrons in an atom or molecule, G. N. Lewis developed Lewis structures. A Lewis structure only counts valence electrons because these are the only ones involved in bonding. To calculate the number of valence electrons, write out the electron configuration of the atom and count up the number of electrons in the highest principle quantum number. The number of valence electrons for neutral atoms equals the group number from the periodic table. Each valence electron is represented by a dot next to the symbol for the atom. Because atoms strive to achieve a full octet of electrons, we place two electrons on each of the four sides of the atomic symbol. Some examples of Lewis structures for atoms are shown in .
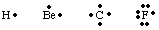
We can create bonds by having two atoms come together to share an electron pair. A bonding pair of electrons is distinguished from a non-bonding pair by using a line between the two atoms to represent a bond, as in the figure below. A lone pair is what we call two non-bonding electrons localized on a particular atom.
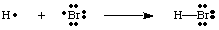
You should note that each atom in the H-Br molecule has a full valence shell. Both the hydrogen and the bromine can count the two electrons in the bond as its own because the electrons are shared between both atoms. Hydrogen needs only two electrons to fill its valence, which it gets through the covalent bond. The bromine has an octet because it has two electrons from the H-Br bond and six more electrons, two in each lone pair on Br.
The deadly gas carbon monoxide, CO, provides an interesting example of how to draw Lewis structures. Carbon has four electrons and oxygen has six. If only one bond were to be formed between C and O, carbon would have five electrons and oxygen 7.
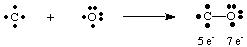
A single bond here does not lead to an octet on either atom. Therefore, we propose that more than one bond can be formed between carbon and oxygen so that we can give each atom an octet of electrons. To complete the carbon and oxygen octets in CO, we must employ a triple bond, denoted by three lines joining the C and O atoms as shown in . A triple bond means that there are six electrons shared between carbon and oxygen. Such multiple bonds must be employed to explain the bonding in many molecules. However, only single, double, and triple bonds are commonly encountered.
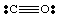
So far we have only dealt with very simple, uncharged molecules. For more complex molecules and molecular ions, it becomes important to keep an accurate count of the number of electrons in the molecule. For example, let us make a Lewis structure for NO2 -. We have five electrons from N, twelve from the oxygen (six from each O), and one extra electron because the molecule has a negative charge. Therefore, NO2 - has a total of eighteen electrons and we should draw the following Lewis structure:
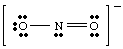
If we had tried to draw the above structure without taking the charge of the ion into account, we could not have produced a full octet around at least one atom. If the ion had been positively charged, as in NO2 +, we would count the electrons as follows: five from N, twelve from O, and minus one due to the charge. The total number of electrons is sixteen for NO2 +, and the molecule will have a Lewis structure different from that of NO2 -because it has a different number of electrons.
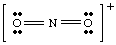
To improve your skills in writing Lewis structures, you should draw as many molecules as possible until you feel confident in your ability to draw Lewis structures.
Formal Charge
When trying to draw the Lewis structures of charged molecules like NO2 - , we encounter the problem of trying to tell where the negative charge is located. Is it on nitrogen or on one of the oxygens? To combat these troubles, chemists have devised the notion of formal charge. Using the Lewis structure and the rules for assigning formal charges, we can assign a formal charge to each atom in a Lewis structure to determine where the charges are located.
Using NO2 - as an example, let's discuss how to determine the formal charges on atoms in molecules. First, we must draw the correct Lewis structure. Then, we break all bonds around each atom giving half the electrons in the bond to each bonded atom. All lone pairs remain on the atom to which they belong in the molecule. This process serves to count the number of electrons each atom has in the molecule and is shown in the figure below.
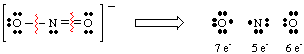
Once we have counted the number of electrons assigned to each atom, we compare the number to the number of valence electrons in the free atom. For example, oxygen has six electrons in the free atom, and it has six electrons in the right-hand oxygen in the . Therefore, the right-hand oxygen has no formal charge because it has the same number of electrons in the NO2 - molecule as it does as an atom. The left-hand, singly bonded oxygen has seven electrons-- one more electron than has the free atom. Therefore, this oxygen has a -1 formal charge because it has one more electron in the molecule than oxygen has as a free atom. The nitrogen has five electrons around it and five valence electrons in the free atom, so the N has no formal charge. In general, formal charge equals the difference between the number of valence electrons of the atom and the number of electrons around the atom in a molecule as assigned by the rules for drawing Lewis structures.
A complete Lewis structure should include both bonding and formal charge information. Therefore, the structure of NO2 - should be drawn as shown in .
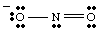
Resonance Structures
While drawing the , you may have noticed that the two oxygens appear to be different. One bears a negative charge and has only one bond to N while the other is neutral and doubly bonded to N. Why should these two oxygens be different? There is absolutely no reason why they should be. It is impossible to make a single Lewis structure that depicts the equivalency of the two oxygens. Instead, we can represent NO2 - as a hybrid of two resonance structures as shown in .
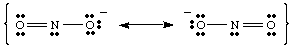
It is important to note that NO2 - is neither one nor the other resonance structure but is the average of the two. A good analogy for resonance structures is found in mixing colors. Green is neither yellow nor blue but is a mixture of the two colors, just as NO2 - is neither of the resonance forms but is a mixture of the two structures.
When more than one reasonable Lewis structure can be drawn for a molecule, the actual structure of the molecule will be a resonance hybrid of the structures.
Exceptions to the Octet Rule
Although the octet rule has allowed us to draw almost every conceivable Lewis structure, there are certain molecules that do not obey the octet rule. In this section, we will point out the most common exceptions.
Boron and aluminum compounds commonly place only six electrons around the metal center. For example, AlH3 has only six electrons on Al. Compounds with less than an octet (or duet for H) of electrons around each atom are called electron deficient. Boron and aluminum compounds are frequently electron deficient while compounds involving most other elements are not. The reason why boron and aluminum can form electron deficient compounds has to do with their low electronegativities. Because both atoms are not very electronegative, they are not terribly unhappy when they have fewer electrons than they require for full octets.
While boron and aluminum can have less than a full octet, some atoms like phosphorous and atoms in period three or below on the periodic table (greater period numbers) can exceed their octets. Try to draw a reasonable Lewis structure for either PF5 or SF6. You should not find it possible to obey the octet rule on either phosphorous or sulfur. Frequently textbooks say that atoms like P and S are able to expand their octets by letting the extra electrons fill their empty 3d orbitals. Your chemistry course may even require you to memorize this "fact". However, this description of the bonding in such compounds is completely false. After you have read Molecular Orbital Theory you should be able to come up with a better reason. The explanation of the expanded octet must wait until then due to is complexity. For now, realize that atoms below period two may expand their octets to accommodate more than eight electrons.
Valence Shell Electron Pair Repulsion Theory
When drawing Lewis structures, only bonding and charge information is available. Such structures tell us absolutely nothing about the real three-dimensional shapes that molecules have. To determine molecular geometry, chemists use Valence Shell Electron Pair Repulsion theory-- abbreviated VSEPR. The VSEPR model makes the reasonable assumption that electron pairs repel each other. Therefore, electron pairs in bonds and lone pairs will want to be oriented as far away from each other as possible. By analyzing all possible combinations of lone pairs and bonding pairs we can predict the structure of any covalent molecule. , , , and show the results of such an analysis. (The tables are broken down into four parts due to the sizes of the images and not because there are any fundamental differences between the tables.) For each, A stands for the central atom, B stands for any other atoms bonded to A, and e stands for the lone pairs on the central atom.
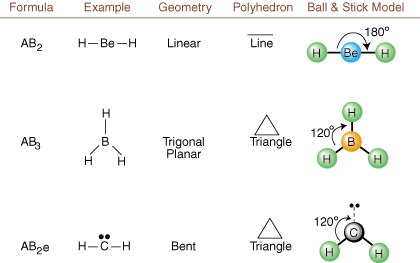
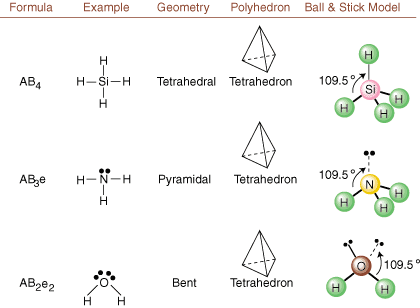
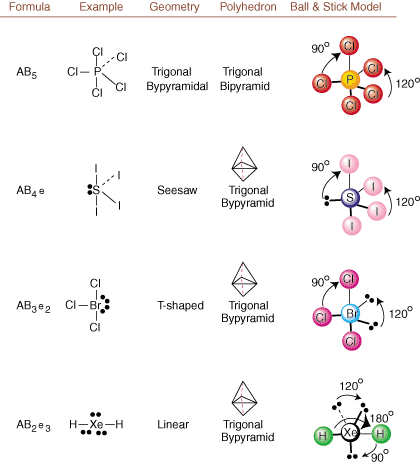
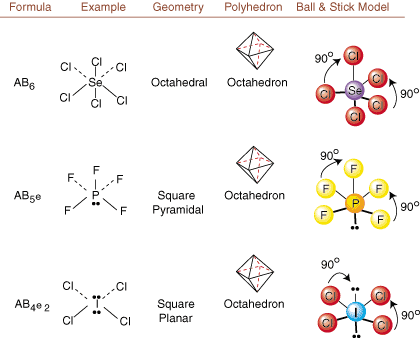
By comparing a Lewis structure to the examples provided in the above figures, you can predict the geometries of many molecules accurately. Note in the tables that the lone pair groups e are placed at positions to minimize interactions with other groups e or B; the lone pairs take up these positions preferentially when you must choose to place either e or B there. For example, for the molecule AB3e2 in , the e's are placed in the equatorial positions where they are at 90 and 120 degree angles from other groups, rather than at the axial positions where they would be restricted to 90 degree angle interactions. We can understand this trend by visualizing the condensed electron density of a lone pair near an atomic center, in comparison with a bonded electron pair in which electron density is distributed between two atoms.
VSEPR theory does not work well for transition metals. To predict their geometries you will need a more advanced treatment of bonding which will not be presented in this SparkNote.
To predict the geometries of polycentric molecules (those with A greater than one), simply use the above tables of geometries to predict the geometry of each center independently of others. For example, to predict the geometry of HOCH2NH2, you need only predict the geometry at oxygen, carbon, and nitrogen. To do so, first draw the Lewis structure as shown in :
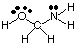
Next, classify the VSEPR type for each atom bonded to more than one other atom using A, B, and e. Oxygen is AB2e2, so it is bent. Carbon is AB4, so it is tetrahedral. Nitrogen is AB3e, so it is pyramidal. Now you can draw the structure of HOCH2NH2 in three dimensions.




No comments:
Post a Comment