Ionic Bonding
As we shall explore in this section on ionic bonding, ionic bonds result from the mutual attraction between oppositely charged ions. They tend to be stronger than covalent bonds due to the coulombic attraction between ions of opposite charges. To maximize the attraction between those ions, ionic compounds form crystal lattices of alternating cations and anions. Ionic compounds are usually formed only between atoms whose difference in electronegativity is large.
In our description of ionic bonding, we will explore the questions of what determines the bond length and bond strength of an ionic bond. We will show that bonds form at particular distances even though the attraction between oppositely charged ions increases strongly with decreasing distance. The opposing strong internuclear repulsion maintains the separation between ions. Bond strength, it will be shown, depends mostly on the charges present on each ion and the distance between them. Small, highly charged ions will form strong bonds while large, minimally charged ions will form weaker bonds.
In our description of ionic bonding, we will explore the questions of what determines the bond length and bond strength of an ionic bond. We will show that bonds form at particular distances even though the attraction between oppositely charged ions increases strongly with decreasing distance. The opposing strong internuclear repulsion maintains the separation between ions. Bond strength, it will be shown, depends mostly on the charges present on each ion and the distance between them. Small, highly charged ions will form strong bonds while large, minimally charged ions will form weaker bonds.
Terms
Ionic Bonding
The Ionic Bond
When a highly electronegative atom and an electropositive one are bonded together, an electron is transferred from the electropositive atom to the electronegative atom to form a cation and an anion, respectively. The cation, being a positively charged ion, is attracted to the negatively charged anion as described by Coulomb's law:
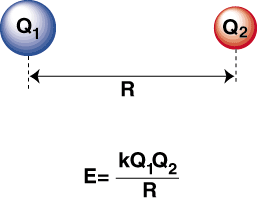
A negative energy means there is an attractive interaction between the particles in the . If the charges on the two ions are opposite in sign, they will attract each other. Conversely, if two charges are similar, they repel each other. Using this knowledge we can construct a graph of energy versus distance for two oppositely charges ions. At large distances, there is a negligible energy of attraction between the two ions, but as they are brought closer together, they are attracted to one another. Coulomb's law may seem to predict that the ions should be as close as possible to achieve a minimal energy state. However, the shows that the ions are actually repelled at small distances. To explain this observation, remember that the ions' nuclei are both positively charged. When the nuclei approach each other, they repel strongly--accounting for the steep rise in potential as the ions get closer than the bond length.
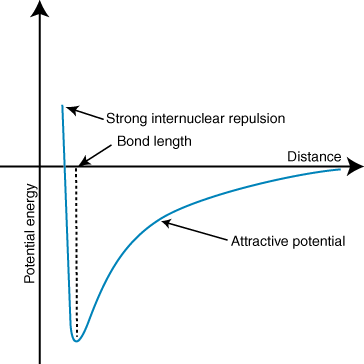
The depth (y-axis) of the minimum in the potential energy curve above represents the bond strength, and the distance (x-axis) at the energy minimum is the bond length. Using Coulomb's law and the bond length, one can actually predict with some accuracy the strength of an ionic bond. Performing a series of these calculations you find that ionic compounds formed by ions with larger charges create stronger bonds and that ionic compounds with shorter bond lengths form stronger bonds.
Crystal Lattices
Ionic compounds do not usually exist as isolated molecules, such as LiCl, but as a part of a crystal lattice--a three dimensional regular array of cations and anions. Ionic compounds form lattices due to the contributing coulombic attractions of having each cation surrounded by several anions and each anion surrounded by several anions. An example of a crystal lattice is shown in :
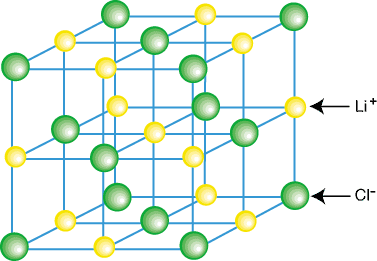
As you can see in the above figure, each lithium ion is surrounded by six chlorine atoms and vice versa. By virtue of the arrangement of the ions in the lattice, the lattice is lower in energy than it would be if the ions were separated into isolated LiCl molecules.




No comments:
Post a Comment