Molecular Orbital Theory
Molecular orbital theory rests on the notion that atomic orbitals are combined to form molecular orbitals. Because electron density from each atom is spread out over the span of the entire molecule, the electrons are lowered in energy. This accounts for the stabilization that occurs during bonding. The amount of the stabilization depends on the amount of overlap between atomic orbitals and the difference in energy between them. Atomic orbitals that overlap effectively produce stable molecular orbitals. One condition for overlap is that the overlapping atomic orbitals must be of similar energies.
The bonding in homonuclear and heteronuclear diatomic molecules will be discussed to show how molecular orbital theory works. Due to the complexities of describing the molecular orbitals in polynuclear molecules, we will introduce the notion of bonding through hybridized atomic orbitals to account for the bonding in such systems.
Molecular orbital theory can give us information about both ionic and covalent molecules and naturally predicts which molecules will be ionic and which will be covalent. It is a powerful and complex tool available to chemists for predicting the properties of molecules. In this SparkNote, only a brief introduction to molecular orbital theory will be given. The interested reader should consult the further reading section accompanying this document to gain access to more detailed treatments.
Terms
Molecular Orbital Theory
Homonuclear Diatomic Molecules
In atoms, as you know, electrons reside in orbitals of differing energy levels such as 1s, 2s, 3d, etc. These orbitals represent the probability distribution for finding an electron anywhere around the atom. Molecular orbital theory posits the notion that electrons in molecules likewise exist in different orbitals that give the probability of finding the electron at particular points around the molecule. To produce the set of orbitals for a molecule, we add together the valence atomic wavefunctions for the bonded atoms in the molecule. This is not as complicated as it may sound. Let's consider the bonding in homonuclear diatomic molecules--molecules of the formula A2.
Perhaps the simplest molecule we can imagine is hydrogen, H2. As we have discussed, to produce the molecular orbitals for hydrogen, we add together the valence atomic wavefunctions to produce the molecular orbitals for hydrogen. Each hydrogen atom in H2 has only the 1s orbital, so we add the two 1s wavefunctions. As you have learned in your study of atomic structure, atomic wavefunctions can have either plus or minus phases--this means the value of the wavefunction y is either positive or negative. There are two ways to add the wavefunctions, either both in-phase (either both plus or both minus) or out-of-phase (one plus and the other minus). shows how atomic wavefunctions can be added together to produce molecular orbitals.
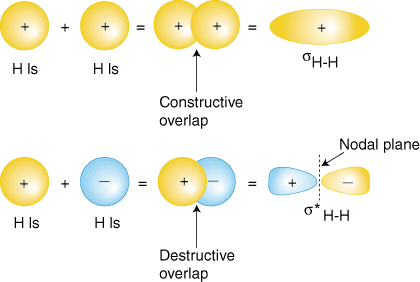
The in-phase overlap combination (top set of orbitals in ) produces a build-up of electron density between the two nuclei which results in a lower energy for that orbital. The electrons occupying the s H-H orbital represent the bonding pair of electrons from the Lewis structure of H2 and is aptly named a bonding molecular orbital. The other molecular orbital produced, s * H-H shows a decrease in electron density between the nuclei reaching a value of zero at the midpoint between the nuclei where there is a nodal plane. Since the s * H-H orbital shows a decrease in bonding between the two nuclei, it is called an antibonding molecular orbital. Due to the decrease in electron density between the nuclei, the antibonding orbital is higher in energy than both the bonding orbital and the hydrogen 1s orbitals. In the molecule H2, no electrons occupy the antibonding orbital.
To summarize these findings about the relative energies of the bonding, antibonding, and atomic orbitals, we can construct an orbital correlation diagram, shown in :
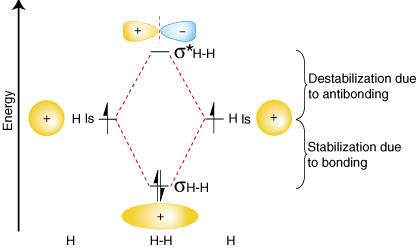
Notice that the orbitals of the separated atoms are written on either side of the diagram as horizontal lines at heights denoting their relative energies. The electrons in each atomic orbital are represented by arrows. In the middle of the diagram, the molecular orbitals of the molecule of interest are written. Dashed lines connect the parent atomic orbitals with the daughter molecular orbitals. In general, bonding molecular orbitals are lower in energy than either of their parent atomic orbitals. Similarly, antibonding orbitals are higher in energy than either of its parent atomic orbitals. Because we must obey the law of conservation of energy, the amount of stabilization of the bonding orbital must equal the amount of destabilization of the antibonding orbital, as shown above.
You may be wondering whether the Lewis structure and the molecular orbital treatment of the hydrogen molecule agree with one another. In fact, they do. The Lewis structure for H2 is H-H, predicting a single bond between each hydrogen atom with two electrons in the bond. The orbital correlation diagram in predicts the same thing--two electrons fill a single bonding molecular orbital. To further demonstrate the consistency of the Lewis structures with M.O. theory, we will formalize a definition of bond order--the number of bonds between atoms in a molecule. The bond order is the difference in the number of electron pairs occupying an antibonding and a bonding molecular orbital. Because hydrogen has one electron pairin its bonding orbital and none in its antibonding orbital, molecular orbital theory predicts that H 2 has a bond order of one--the same result that is derived from Lewis structures.
To demonstrate why it is important to take the number of antibonding electrons into account in our bond order calculation, let us consider the possibility of making a molecule of He2. An orbital correlation diagram for He2 is provided in :
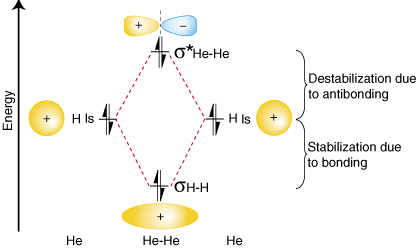
From the orbital correlation diagram above you should notice that the amount of stabilization due to bonding is equal to the amount of destabilization due to antibonding, because there are two electrons in the bonding orbital and two electrons in the antibonding orbital. Therefore, there is no net stabilization due to bonding so the He2 molecule will not exist. The bond order calculation shows that there will be a bond order of zero for the He2 molecule--exactly what we should predict given that helium is a noble gas and does not form covalent compounds.
Both hydrogen and helium only have 1s atomic orbitals so they produce very simple correlation diagrams. However, we have already developed the techniques necessary to draw a correlation diagram for a more complex homonuclear diatomic like diboron, B2. Before we can draw a correlation diagram for B2, we must first find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then, we rank them in order of increasing energy. Each boron atom has one 2s and three 2p valence orbitals. Due to the great difference in energy between the 2s and 2p orbitals, we can ignore the overlap of these orbitals with each other. All orbitals composed primarily of the 2s orbitals will be lower in energy than those comprised of the 2p orbitals. shows the process of creating the molecular orbitals for diboron by combining orbitals of atomic boron. Note that the orbitals of lowest energy have the most constructive overlap (fewest nodes) and the orbitals with the highest energy have the most destructive overlap (most nodes).
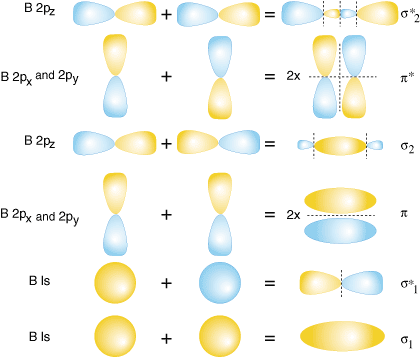
Notice that there are two different kinds of overlap for p-orbitals--end-on and side-on types of overlap. For the p-orbitals, there is one end-on overlap possible which occurs between the two pz. Two side-on overlaps are possible--one between the two px and one between the two p y. P-orbitals overlapping end-on create s bonds. When p-orbitals bond in a side-on fashion, they create p bonds. The difference between a p bond and a s bond is the symmetry of the molecular orbital produced. s bonds are cylindrically symmetric about the bonding axis, the z-direction. That means one can rotate the s bond about the z-axis and the bond remains the same. In contrast, p bonds lack that cylindrical symmetry and have a node passing through the bonding axis.
Now that we have determined the energy levels for B2, let's draw the orbital correlation diagram ():
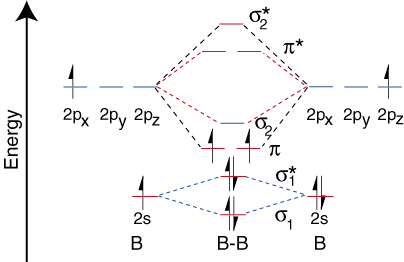
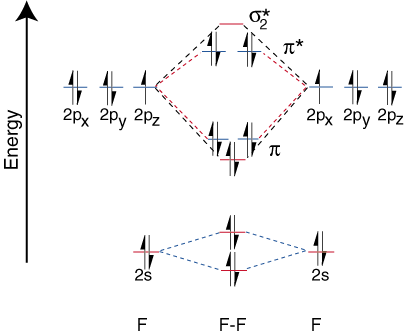
The orbital correlation diagram for diboron, however, is not generally applicable for all homonuclear diatomic molecules. It turns out that only when the bond lengths are relatively short (as in B2, C2, and N2) can the two p-orbitals on the bonded atoms efficiently overlap to form a strong p bond. Some textbooks explain this observation in terms of a concept called s-p mixing. For any atom with an atomic number greater than seven, the p bond is less stable and higher in energy than is the s bond formed by the two end-on overlapping p orbitals. Therefore, the following orbital correlation diagram for fluorine is representative of all homonuclear diatomic molecules with atomic numbers greater than seven.
Heteronuclear Diatomic Molecules
To draw the correlation diagrams for heteronuclear diatomic molecules, we face a new problem: where do we place the atomic orbitals on an atom relative to atomic orbitals on other atoms? For example, how can we predict whether a fluorine 2s or a lithium 2s orbital is lower in energy? The answer comes from our understanding of electronegativity. Fluorine is more electronegative than lithium. Then electrons are more stable, i.e. lower in energy, when they are lone pairs on fluorine rather than on lithium. The more electronegative element's orbitals are placed lower on the correlation diagram than those of the more electropositive element. illustrates this point:

Since lithium only has one occupied valence orbital, only one bonding and one antibonding orbital are possible. Furthermore, the electrons in orbitals on F that cannot bond with Li are left on F as lone pairs. As you can see, the electrons in the Li-F s bond are quite close in energy to fluorine's 2p orbitals. Then the bonding orbital is primarily composed of a fluorine 2p orbital, so the M.O. diagram predicts that the bond should be polarized toward fluorine--exactly what is found by measuring the bond dipole. Such an extreme polarization of electron density towards fluorine represents a transfer of an electron from lithium to fluorine and the creation of an ionic compound.
The construction of other heteronuclear diatomic orbital correlation diagrams follows exactly the same principles as those we employed for LiF. To see more examples of such diagrams, consult your favorite chemistry textbook.
Bonding in Polyatomic Molecules
As you can imagine, to describe the bonding in polyatomic molecules, we would need a molecular orbital diagram with more than two dimensions so we could describe the bonds both between the central atom and each terminal atom and between the terminal atoms themselves. Such diagrams are impractically difficult to draw or require complex methods to collapse such multidimensional figures into two dimensions. Instead we will describe a simple yet powerful method to describe the bonding in polyatomic molecules called hybridization. By adding together certain atomic orbitals, we can produce a set of hybridized atomic orbitals that have the correct shape and directionality to account for the known bond angles in polyatomic molecules. Hybrid orbitals describe the bonding in polyatomic molecules one bond at a time.
From the geometry of the molecules, as predicted by VSEPR, we can deduce the hybridization of the central atom. Linear molecules are sp hybridized. Each hybrid orbital is composed of a combination of an s and a p orbital on the central atom. The other geometries are produced by the proper mixture of atomic orbitals. Molecules based on a triangle are sp2hybridized. Tetrahedrally based molecules are sp3 hybridized. Trigonal bipyramidally based molecules are dsp3 hybridized. Octahedrally based molecules are d2sp3 hybridized.
To illustrate how hybrid orbitals are used to describe the bonding in polyatomic molecules, we will examine the bonds that form water, H2O. Water is AB2e2, therefore, its geometry is based on a tetrahedron, and it is sp3 hybridized. Two sp3 hybrid orbitals on oxygen with one electron each can form a bond with the singly occupied 1s orbitals on the hydrogen atoms. The remaining two sp3 hybrid orbitals on oxygen each have two electrons in them and are, therefore, lone pairs. A model of the bonding in water is shown in :
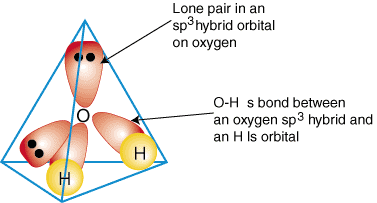
To produce hybrid bonding descriptions of any compound, first decide what is the hybridization of the central atom based on its geometry. Next, form bonds between the hybrid or atomic orbitals on terminal atoms and the central atom. Finally, check to make sure that your bonding description agrees with the Lewis structure in the number of bonds formed and the number of lone pairs.




No comments:
Post a Comment