Introduction
Fundamental to our understanding of the chemical bond are the experimentally measured properties of a bond--its bond length, bond strength, and bond dipole. From bond dipole data we will derive the concept of electronegativity--that some atoms have a greater power to attract electrons than have others.
These ideas will allow us to discover two types of bonding--ionic and covalent. Compounds with large electronegativity differences tend to have unusually strong bonds. This can be rationalized by proposing that an electron is given from the more electropositive atom to the more electronegative one. This situation creates an anion and a cation that are bonded together primarily by electrostatic attraction between the oppositely charged ions. For bonded atoms with similar electronegativities, the sharing of electrons between the nuclei produces a covalent bonding interaction.
These ideas will allow us to discover two types of bonding--ionic and covalent. Compounds with large electronegativity differences tend to have unusually strong bonds. This can be rationalized by proposing that an electron is given from the more electropositive atom to the more electronegative one. This situation creates an anion and a cation that are bonded together primarily by electrostatic attraction between the oppositely charged ions. For bonded atoms with similar electronegativities, the sharing of electrons between the nuclei produces a covalent bonding interaction.
Properties of Chemical Bonds
Why Make a Bond?
Why should atoms bond at all? In nature we find that some elements like He, Ne, and Ar are never found bonded to other atoms whereas most other elements are only found bonded to other elements. What makes the noble gases so special? The answer lies in their closed shell electron configurations. Because the valence shell of a noble gas is completely full, it cannot accept another electron into the shell. The nucleus is positively charged and pulls on the electron, so the loss of an electron from a noble gas is unfavorable. Therefore, like real nobility, the noble gases do not want to do anything at all--that is, noble gases are unreactive because they have filled valence shells.
Any element other than a noble gas has an open shell configuration, which is unstable relative to the configuration of a noble gas. Non-noble atoms react to form bonds in an attempt to achieve a closed shell electron configuration. For example, when a lithium atom and a fluorine atom meet, as shown in , lithium can achieve a noble gas configuration, 1s2, by donating an electron to fluorine which also achieves the noble gas configuration 1s22s22p6:
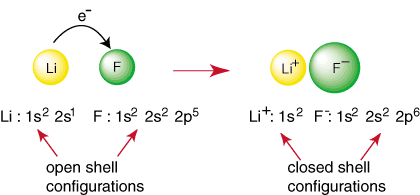
The above reaction represents the formation of an ionic bond. The negatively charged anion, F, and the positively charged cation are held together in the bond by the attraction of unlike charges as dictated by Coulomb's law. You may have asked yourself why two fluorine atoms don't come together to perform the following reaction:
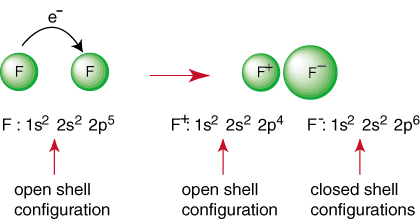
Even though the reaction may appear to be favorable because of its production of a closed shell species, there is a way to have both F atoms achieve a noble gas configuration. By sharing their electrons, each fluorine atoms can have a complete octet in its valence shell. Such a sharing of electrons is called a covalent bond and will be discussed in depth in aseparate section.
Properties of a Bond
The way bond properties were chosen to characterize bonds have a historical basis. Scientists made their first rational attempts to describe bonding by looking at data they could collect about bonds. We too will look at the experimental data on bonds to try to analyze bonding.
Perhaps the most useful aspect to know of a bond is its strength. Weak bonds are easily broken and molecules with such bonds are fairly reactive. Conversely, strong bonds are difficult to break and give rise to stable molecules. Therefore, it is sensible to define bond strength as the amount of energy needed to break a chemical bond. Trends in bond strength show that homoatomic bonds (those formed between atoms of the same element) tend to be strong. But going across a row in the periodic table, the trend in bond strength may not be regular. For example, period 2 elements have the following strength order: Li-Li > Be-Be <> O-O > F-F. This irregular trend is repeated in period 3 homoatomic bonds. If we look at bond strength data, we also notice that the Li-F bond is several times stronger than the F-F bond or the Li-Li bond. It is not important for you to memorize such trends. We use them to show that whatever theory of covalent bonding we propose must account for these observations.
Bond lengths follow the expected trend that bonds between larger atoms are longer and bonds between smaller atoms are smaller. What is surprising is that bond strength and bond length are inversely related--a short bond is generally stronger than a long one. Another unexpected piece of bond length data shows that there are three common bond lengths for C-C bonds. Our bonding theory must also predict the above trends in bond length.
Bond dipole data provides at least a partial answer for several of the above observations. Because F is a more electronegative atom than C, the electrons in a C-F bond will be polarized toward F. The fluorine atom then acquires a large partial negative charge (δ-) and the carbon atom a large partial negative charge (δ+) as shown in :
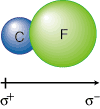
The crossed arrow underneath the C-F bond indicates that there is a partial positive charge located on C and a partial negative charge located on F. The charge separation leads to a coulombic attraction between the two atoms in the bond and makes the C-F bond stronger than either the C-C or F-F bonds, which have no bond dipoles. This stronger bond will have the nuclei closer than the sum of their atomic radii due to the coulombic attraction between the oppositely charged ends of the molecule.
In the next section we will take a closer look at ionic bonding.




No comments:
Post a Comment