Summary
This first, short introductory SparkNote to Stoichiometry is meant to give you a very basic and general understanding of what stoichiometry is. It also introduces the most important concept of stoichiometry: the mole.
Terms
Overview
What is Stoichiometry?
Stoichiometry is at the heart of the production of many things you use in your daily life. Soap, tires, fertilizer, gasoline, deodorant, and chocolate bars are just a few commodities you use that are chemically engineered, or produced through chemical reactions. Chemically engineered commodities all rely on stoichiometry for their production.
But what is stoichiometry? Stoichiometry is the calculation of quantities in chemical equations. Given a chemical reaction, stoichiometry tells us what quantity of each reactant we need in order to get enough of our desired product. Because of its real-life applications in chemical engineering as well as research, stoichiometry is one of the most important and fundamental topics in chemistry.
Introduction to the Mole
Which weighs more, 100 pounds of feathers or 100 pounds of bowling balls? You've probably heard this riddle before. Obviously they both weigh the same since I told you I have 100 pounds of each. But if I have 100 pounds of bowling balls and 100 pounds of feathers, do I have more feathers or more bowling balls? The quantities of feathers and bowling balls would not be equal. An individual feather weighs a lot less than a bowling ball. It would take only about 10 bowling balls to get 100 pounds whereas it would take a LOT more feathers.
When you measure quantities in moles, however, the situation is exactly opposite from when you measure according to weight. A mole is defined as the amount of a substance. More specifically, there are 6.02×1023particles in a mole of substance. Therefore, if you had 1 mole of feathers and 1 mole of bowling balls, you would have 6.02×1023 feathers and6.02×1023 bowling balls. Now suppose you were asked the question, "Which weighs more, 100 moles of feathers or 100 moles of bowling balls?" The answer this time would be the bowling balls. Although there is an equal number of both feathers and bowling balls, an individual bowling ball weighs much more than an individual feather, and so an equal number of bowling balls would weigh more than an equal number of feathers.
Now, let's return to the number 6.02×1023 . This number is known as Avogadro's number and you should definitely commit it to memory. You are probably wondering why it's so large, and it does indeed look intimidating without the exponential notation:
| 6.02×1023 = 60, 200, 000, 000, 000, 000, 000, 000, 000 |
Although you will never have a mole of bowling balls, you will soon be calculating moles of compounds, molecules, atoms, and ions. These representative particles are extremely and incredibly small. That is why there are so many particles in a mole of substance. When you appreciate just how small these particles are, 6.02×1023 stops seeming like such a crazy number.
Summary
In this lesson you will learn all about conversion factors and how to use them. You will use them to solve general problems and then to solve your first stoichiometry problems. Most importantly, you will learn the four steps necessary to solve ANY stochastic problem:
- Balance the equation
- Convert units of given substance to moles
- Find moles of wanted substance using mole ratio
- Convert moles of wanted substance to desired units
Only move on to the next lesson after you:
- Completely understand the steps involved in stoichiometric problems.
- Memorize the three all-important conversion factors in the formulas section of the lesson.
Terms
Formulae
Conversion Factor =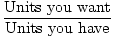 |
Moles =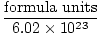 |
moles =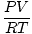 |
Moles =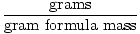 |
Conversion Factors
The easiest way to do stoichiometric calculations involves using conversion factors. A conversion factor is a ratio (or fraction) which represents the relationship between two different units. A conversion factor is ALWAYS equal to 1. Here are some examples of conversion factors:
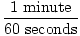 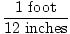 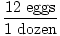 |
All these conversion factors are equal to 1. If it doesn't seem obvious at first, think about it for a second. Realize that 1 minute is equivalent to 60 seconds. Simply replace 1 minute in the fraction with its equivalent 60 seconds and it becomes clear that 60 seconds / 60 seconds = 1. Numerator and denominator are equivalent; they are just expressed differently.
As you can see it is extremely important to keep track of your units when using conversion factors. Without units, the first fraction would be 1 / 60. This is not equal to 1 and could very easily lead to wrong answers.
Furthermore, when you use units, you make it very easy to check your work. For example, perhaps you are trying to find out how many dozen eggs you have to buy to make three cakes. If you're getting an answer of 12 dozen eggs you might want to check your work. Could you even fit 12 of those cartons in your refrigerator? If you look back on your calculations you may immediately see the incorrect conversion factor: 1 egg / 12 dozen. It is easy to see that this is where the error occurred since this does NOT equal 1.
How do you use Conversion Factors?
We all know from elementary school math that if you multiply any quantity by 1 you get the same quantity back. You can do this as many times as you want. For example, 2×1 = 2 , and 18×1×1×1 = 18 .
Multiplication by 1 is what you do whenever you do a problem involving conversion factors. The best way to explain how to solve using conversion factors is to work through some simple examples.
Problem: How many days are there in 3 years? (Assume none of these years are leap years)
Solution: Here we basically want to convert years to days. Our conversion factor is:
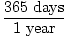 |
Since this is equivalent to 1, multiplication of this ratio with our original value will only change its units and not its magnitude. Therefore:
3 years×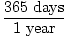 = 1, 095 days = 1, 095 days |
Notice that years is on the bottom of the conversion factor. This is VERY important. You always want to have the units of what you currently have on the bottom of the conversion factor and the units you want on the top.
Conversion Factor = 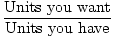 |
In this case we are multiplying our conversion factor by years. We therefore put years on the bottom of the conversion factor. When multiplied together, the resulting fraction has years in both numerator AND denominator. These units can now "cancel each other out". How? You might want to think about it like this. When you see the fraction 2 / 2, you cancel the 2s in both numerator and denominator. You can do the same thing with units.
When doing any type of problem involving conversion factors, feel free to draw a line through any unit you see on the top and bottom of the fraction to make it visually obvious that the units cancel.
3 years×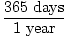 = 1, 095 days = 1, 095 days |
Canceling units in this way makes it much easier to check your work. The units you want in your answer should be the only unit not to cancel. If your calculations yield other units, which cannot be cancelled, you have made a mistake likely resulting from a missing conversion factor or an upside down conversion factor that needs to be flipped.
Stoichiometric Calculations
Applying Conversion Factors to Stoichiometry
Now you're ready to use what you know about conversion factors to solve some stoichiometric problems in chemistry. Almost all stoichiometric problems can be solved in just four simple steps:
- Balance the equation.
- Convert units of a given substance to moles.
- Using the mole ratio, calculate the moles of substance yielded by the reaction.
- Convert moles of wanted substance to desired units.
Let's begin our tour of stoichiometry by looking at the equation for how iron rusts:
| Fe + O2→Fe2O3 |
Step 1. Balancing the Equation
The constituent parts of a chemical equation are never destroyed or lost: the yield of a reaction must exactly correspond to the original reagents. This fact holds not just for the type of elements in the yield, but also the number. Given our unbalanced equation:
| Fe + O2→Fe2O3 |
This equation states that 1 iron (Fe) atom will react with two oxygen (O) atoms to yield 2 iron atoms and 3 oxygen atoms. (The subscript number, such as the two inO2 describe how many atoms of an element are in a molecule.) This unbalanced reaction can't possibly represent a real reaction because it describes a reaction in which one Fe atom magically becomes two Fe atoms.
Therefore, we must balance the equation by placing coefficients before the various molecules and atoms to ensure that the number of atoms on the left side of the arrow corresponds exactly to the number of elements on the right.
| 4Fe +3O2→2Fe2O3 |
Let's count up the atoms in this new, balanced version of the reaction. On the left of the arrow we have 4 atoms of iron and 6 atoms of oxygen (since 3×2 = 6 ). On the right we also have 4 iron (since 2×2 = 4 ) and 6 oxygen ( 2×3 = 6 ). The atoms on both sides of the equation match.
The process of balancing an equation is basically trial and error. It gets easier and easier with practice. You will likely start to balance equations almost automatically in your mind.
Step 2. Converting Given Units of a Substance to Moles
The process of converting given units into moles involves conversion factors. Below we will provide the most common and important conversion factors to convert between moles and grams, moles and volumes of gases, moles and molecules, and moles and solutions. These conversion factors function in the same way as those discussed in the previous section Note also that though these conversion factors focus on converting from some other unit to moles, they can also be turned around, allowing you to convert from moles to some other unit.
Converting from Grams to Moles
The gram formula mass of a compound (or element) can be defined as the mass of one mole of the compound. As the definition suggests, it is measured in grams/mole and is found by summing the atomic weights of every atom in the compound. Atomic weights on the periodic table are given in terms of amu (atomic mass units), but, by design, amu correspond to the gram formula mass. In other words, a mole of a 12 amu carbon atom will weigh 12 grams.
The gram formula mass can be used as a conversion factor in stoichiometric calculations through the following equation:
Moles = 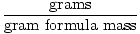 |
Gram formula mass is also known as GFM. You may also see the term gram molecular mass, abbreviated GMM. This term is often used instead of GFM when the substance is molecular and not ionic. However, only the terminology is different, GMM is used in the same way as GFM. Therefore, I will use the catch- all term GFM in this study guide.
Converting between Volume of a Gas and Moles
The Ideal Gas law, discussed at length in the Sparknote on Gases, provides a handy means of converting between moles and a gas, provided you know certain qualities of that gas. The Ideal Gas Law is PV = nRT , withn representing the number of moles. If we rearrange the equation to solve for n , we get:
n = 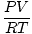 |
with P representing pressure in atm, V representing volume in liters, T representing temperature in Kelvins, and R the gas constant, which equals .0821 L-atm/mol-K. Given P , V , and T , you can calculate the number of moles of substance in a gas.
In those instances when a problem specifies that the calculations are to be made at STP (Standard Temperature and Pressure; P = 1 atm, T = 273 K)), the problem becomes even simpler. At STP, a mole of gas will always occupy 22.4 L of volume. If you are given a volume of a gas at STP, you can calculate the moles in that gas by calculating the volume you are given as a fraction of 22.4 L. At STP, 11.2 L of a gas will be .5 moles; 89.6 L of gas will be 4 moles.
Converting between Individual Particles and Moles
Avogadro's Number provides the conversion factor for moving from number of particles to moles. There are 6.02×1023 formula units of particles in every mole of substance, with formula unit describing the substance we are looking at, whether it is a compound, molecule, atom, or ion. A formula unit is the smallest unit of a substance that still retains that substance's properties and is the simplest way to write the formula of the substance without coefficients. Some representative formula units are listed below.
- Compounds: Cu2S , NaCl
- Molecules: N2 , H2
- Atoms: Fe, Na
- Ions: Na+(aq) , Cl-(aq)
Moles = 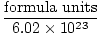 |
Converting between Solutions and Moles
Solutions are discussed in much greater detail in the series of Solutions SparkNotes. But it is possible, and fairly easy to convert between the measures of solution (molarity and molality) and moles.
Molarity is defined as the number of moles of solute divided by the number of liters of solvent. Rearranging the equation to solve for moles yields:
Moles = molarity × liters of solution
MolaLity is defined as the number of moles of solute divided by the number of kilograms of solvent. Rearranging the equation to solve for moles yields:
Moles = molality × kilograms of solution
Using the Mole Ratio to Calulate Yield
Before demonstrating how to calculate how much yield a reaction will produce, we must first explain what the mole ratio is.
The Mole Ratio
Let's look once again at our balanced demonstration reaction:
| 4Fe +3O2→2Fe2O3 |
The coefficients in front of iron, oxygen, and iron (III) oxide are ratios that govern the reaction; in other words, these numbers do not demand that the reaction can only take place with the presence of exactly 4 moles of iron and 3 moles of oxygen, producing 2 moles of iron (III) oxide. Instead, these numbers state the ratio of the reaction: the amount of iron and oxygen reaction together will follow a ratio of 4 to 3. The mole ratio describes exactly what its name suggests, the molar ratio at which a reaction will proceed. For example, 2 moles of Fe will react with 1.5 moles of O2 to yield 1 mole of Fe2O3 . Alternatively, 20 moles of Fe will react with 15 moles ofO2 to yield 10 moles of Fe2O3 . Each of these examples of the reaction follow the 4:3:2 ratio described by the coefficients.
Now, with a balanced equation, the given units converted to moles, and our understanding of the mole ration, which will allow us to see the ratio of reactants to each other and to their product, we can calculate the yield of a reaction in moles. Step 4 demands that we be able to convert from moles to back to the units requested in a specific problem, but that only involves turning backwards the specific converstion factors described above.
Sample Problems
Problem: Given the following equation at STP:
| N2(g) + H2(g)→NH3(g) |
Determine what volume of H2(g) is needed to produce 224 L of NH3(g).
Solution:
br> Step 1: Balance the equation.
| N2(g) + 3H2(g)→2NH3(g) |
Step 2: Convert the given quantity to moles. Note in this step, 22.4 L is on the denominator of the conversion factors since we want to convert from liters to moles. Remember your conversion factors must always be arranged so that the units cancel.
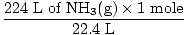 = 10 moles of NH3(g) = 10 moles of NH3(g) |
Step 3: mole ratio.
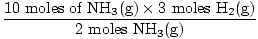 = 15 moles H2(g) = 15 moles H2(g) |
Step 4, convert to desired units:
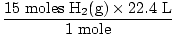 = 336 L H2(g) = 336 L H2(g) |
Now for a more challenging problem:
Given the following reaction:
| 2H2S(g) + O2(g)→SO2(g) + 2H2O(s) |
How many atoms of oxygen do I need in order to get 18 g of ice?
Solution
Step 1. The equation is partially balanced already, but let's finish the job.
| 2H2S(g) +3O2(g)→2SO2(g) + 2H2O(s) |
Step 2, convert to moles:
1 formula unit of H2O has 2 atoms of H and 1 atom of O
The atomic mass of H is 1 gram/mole
Atomic mass of O = 16 grams/mole
GFM of H2O(s) = 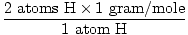 + + 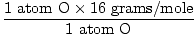 = 18 grams / mole = 18 grams / mole |
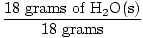 ×1 mole = 1 mole of H2O(s) ×1 mole = 1 mole of H2O(s) |
Step 3, mole ratio:
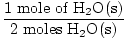 ×3 moles O2(g) = 1.5 moles O2(g) ×3 moles O2(g) = 1.5 moles O2(g) |
Step 4, convert to desired units:
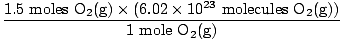 = 9.03×1023 molecules O2(g) = 9.03×1023 molecules O2(g) |
Is this the answer? No. The question asks for ATOMS of oxygen. There are two atoms of oxygen in each molecule of O2(g).
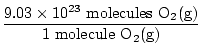 ×2 atoms O = 1.806×1024 atoms O ×2 atoms O = 1.806×1024 atoms O |
Now we're done. Note how important it was to write out not only your units, but what substance you're currently working with throughout the problem. Only a brief check was needed to ascertain if we were really answering the given question. Always check to make sure you have answered the correct question.




No comments:
Post a Comment