The Kinetic Molecular Theory
While the ideal gas law deals with macroscopic quantities of gas, the kinetic molecular theory shows how individual gas particles interact with one another. The kinetic molecular theory contains a number of statements compatible with the assumptions of the ideal gas law. It is worthwhile to list them here:
- Molecules are point masses (they have no volume)
- Gas molecules exert no force on each other unless they collide
- Collisions of molecules with each other or the walls of the container do not decrease the energy of the system
- The molecules of a gas are in constant and random motion
- The temperature of a gas depends on its average kinetic energyavg(1/2mv 2) = 3/2kT . In other words, the energy of an ideal gas is entirely kinetic.
Terms and FormulaeTermsBoltzmann constant - A constant, k , involved in the equation for average velocity. k = 1.38×10-23 J/K Diffusion - Diffusion is the spread of one substance through another. Effusion - Effusion is the rate at which a gas passes through a small hole into a vacuum. Kinetic energy - E k = 1/2mv 2 Kinetic molecular theory - A theory that models the interaction between individual gas molecules. See the summary for more details. Maxwell-Boltzmann speed distribution - The distibution attained when molecule speed is set against the number of molecules sharing that speed. Mean free path - The mean distance a molecule travels before it impacts another molecule; given the huge number of collisions in a gas, the mean free path is vastly smaller than any typical room or container. The equation for mean free path:
m p - The most probable velocity at which the most molecules in a gas travel. The formula for most probable velocity is:
Root mean square velocity - An equation to measure the typical velocity of molecules in a gas.
Formulae
Kinetic Molecular Theory and Its ApplicationsKinetic Molecular TheoryThe most immediately useful bit of information you can pull from the definition of the kinetic molecular theory provided in the summary is that the average kinetic energy of a gas is proportional to the absolute temperature.
@@Equation @@ has a number of very serious implications. First of all, any two gases at the same temperature will have the same kinetic energy. Remember that kinetic energy Ek = 1/2mv 2 , and that average kinetic energy Here's where things get complicated. After some mathematical maneuvering we find a more exact expression for the average velocity $\overline{v}$:
k is the Boltzmann constant. Think of the Boltzmann constant as the gas constant R for individual molecules. Analogously, m is the mass per molecule, just as M is the mass per mole. If you multiply k by Avogadro's number, you'll get R . Let's take a breather. In order to keep things simple, I have refrained from including derivations. If you are at all mathematically inclined, however, I suggest that you take a look in a good physics book (look under statistical mechanics or ideal gases) at the derivations of Let's get back into the fray. There are two other characterizations of v that you should know: the most probable velocity $v_p$ and the root mean square velocity $v_{\mbox{rms}}$. The most probable velocity is exactly what it sounds like: the velocity at which the greatest number of molecules in a gas travel. It can be expressed mathematically:
The root mean square velocity, which measures the typical velocity of molecules in a gas, is slightly tricky. To derive its value, find the square root of the mean of the squares of the average velocity. It is easier to understand mathematically:
Make sure that you see that v rms = 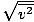 , NOT v rms = , NOT v rms = 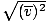 . The latter equation reduces to v rms = . The latter equation reduces to v rms = 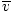 , which is not the case. v rms requires the mean of the squares of the velocities. Square the velocities first, then take their mean. , which is not the case. v rms requires the mean of the squares of the velocities. Square the velocities first, then take their mean.When solving for these values of v , be sure to reduce all variables to SI units. M is particularly insidious--it must be in kg/m 3 if all the other units are SI. Maxwell-Boltzmann Speed DistributionsYou'll often see the range of speeds plotted against the number of molecules on a Maxwell-Boltzmann speed distribution. Plotting the values of
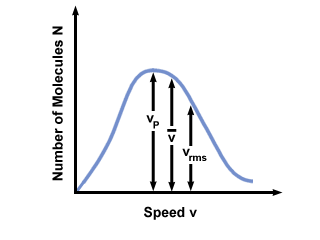 Figure %: Maxwell-Boltzmann Speed Distribution 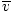 ). The difference between v pand v rms is even more exaggerated because it involves the mean of squares. ). The difference between v pand v rms is even more exaggerated because it involves the mean of squares.A Maxwell-Boltzmann speed distribution changes with temperature. As discussed with the kinetic molecular theory, higher temperatures lead to higher velocities. Thus the distribution of a gas at a hotter temperature will be broader than it is at lower temperatures. 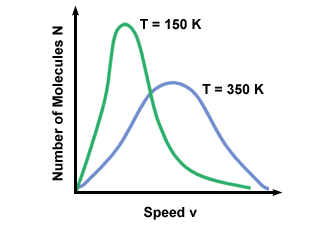 Figure %: Dependence of Maxwell-Boltzmann speed distribution on Temperature The Maxwell-Boltzmann speed distribution also depends on the molecular mass of the gas. Heavier molecules have, on average, less kinetic energy at a given temperature than light molecules. Thus the distribution of lighter molecules like H 2 is much broader and faster than the distribution of a heavier molecule like O 2 : 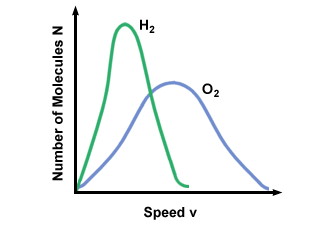 Figure %: Dependence of Maxwell-Boltzmann speed distribution on Molecular Mass Diffusion and Mean Free PathDiffusion is the spread of one substance through another. The fact that molecules collide when they diffuse is the reason why it takes a considerable amount of time for a gas to travel from one place to another. Think, for example, of a smell released at one point in the room. Because gas molecules move at such fast velocities, if there were no collisions the smell would fill the room instantly. The collision between gas molecules makes the calculation of the rate of diffusion difficult. Instead, we'll focus on the mean free path. The mean free path λ is the mean distance a molecule travels before it impacts another molecule; given the huge number of collisions in a gas, the mean free path is vastly smaller than any typical room or container. The mean free path is calculated with the following formula:
N is the total number of molecules present. The rate of collisions is simply v rmsdivided by the mean free path:
EffusionEffusion is the rate at which a gas passes through a small hole into a vacuum. The rate of effusion of a gas is directly proportional to v rms :
|
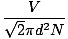
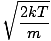 =
=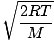
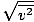
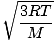
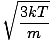
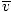 =
=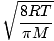 =
=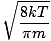
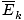 âàùT
âàùT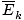 = 1/2m
= 1/2m 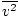
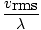
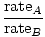 =
= 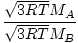 =
= 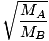




No comments:
Post a Comment